Peroxide Curing in Silicone Manufacturing
An important part of the silicone manufacturing process is the kind of catalyst used to create the product. During production, a catalyst is added to make the silicone cure, which hardens and sets the final product. Typically, a peroxide catalyst is the cheapest and most common catalyst used to manufacture silicone gaskets, o-rings, strips and cords in the construction sector. The kind of catalyst used can have a significant impact on the product’s appearance, physical behaviour and chemical properties. Thus, it is important to understand how different catalysts can affect the properties of peroxide cured silicone. At Jehbco, we work with our clients to ensure that the ideal catalyst is used to deliver the best silicone product for your application.

Silicone Chemistry with Jehbco Silicones
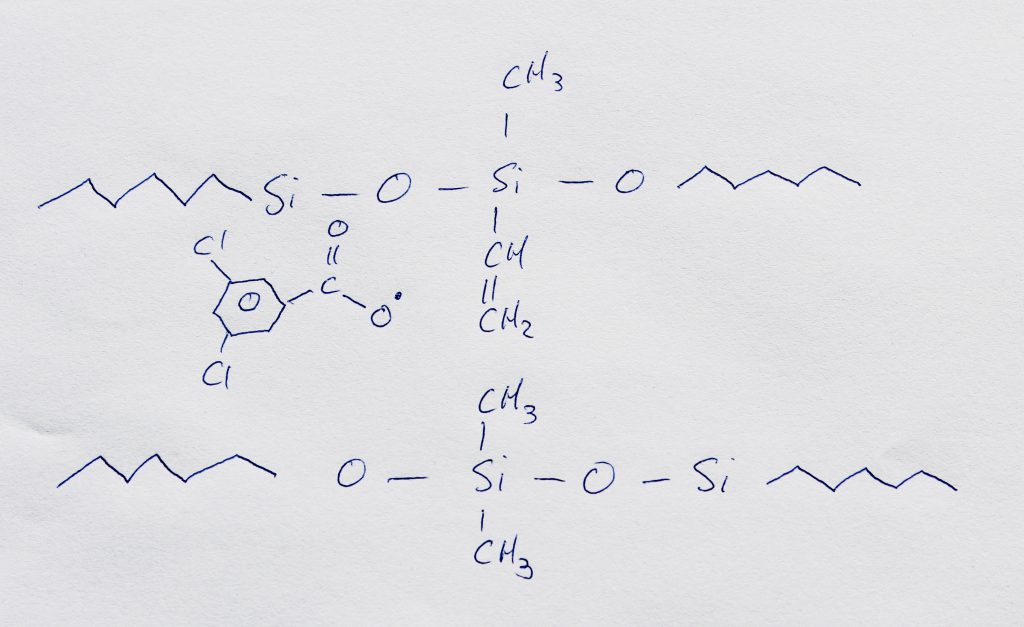
By definition, a catalyst is a substance that facilitates a forward chemical reaction without being depleted as the reaction takes place. The first step in a peroxide cure is the spontaneous formation of peroxide radicals. These peroxide radicals then break double bonds in the silicone chain structure. The site where the double bond is broken is free to form a single bond with various chemical groups on another silicone chain through a variety of reaction pathways. The bonds that stretch between silicone chains forms a cross-link. This cross-linking between silicone chains is what causes an increase in toughness in the curing process.
Reaction Chain with Jehbco Silicones
Above: An example reaction to illustrate the formation of a cross-link between two separate silicone chains. The peroxide catalyst allows the double bond to be broken.
A side effect of peroxide curing is a phenomenon known as ‘blooming’. This occurs when the left-over catalyst migrates to the surface of the silicone, forming a powdery white substance or ‘bloom’. This bloom primarily consists of volatile organic acids. Due to this phenomenon, peroxide cured catalysts undergo a post-curing process where they are heated in a controlled and ventilated environment at 200OC for 4 hours. This process removes all of the catalyst and other extractables from the silicone, leaving behind a pure silicone rubber product. However, peroxide cured catalyst unsuitable for food grade applications, as any minuscule amount of extractables left in the silicone is undesirable due to contamination concerns.
The addition of around 1-1.5% w/w of peroxide catalyst to a silicone mixture is enough to facilitate cross-linking. The small amount of catalyst required, as well as the lower cost of peroxide catalyst, is what makes it useful and effective for silicone manufacturing. Peroxide cured silicones are typically more rigid than platinum cured silicones, making them an ideal material for construction or transportation applications. Peroxide cured silicones are often more opaque and sometimes darker than platinum cured silicone.
Ultimately, cross-linked, peroxide cured silicone is a strong, versatile material that is used to manufacture a variety of products. Deciding whether or not peroxide cured silicone is best for your application can depend on a number of factors, but it is most important to think about the cost of the product, product appearance and whether or not food grade silicone is required. At Jehbco we offer both peroxide and platinum cured silicones for an extensive range of applications and industries.
For further information or advice about which silicone rubber best suits your application, please review the Jehbco website www.jehbco.com.au, and contact us with any questions.


